0755-2650 6355
Follow Us


- 中
0755-2650 6355


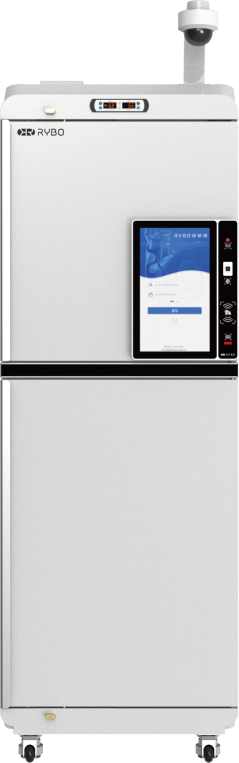
Through the construction of intelligent equipment and information platform, it seamlessly connects with the hospital's clinical trial drug management system, establishes a full-process digital management model, realizes the dynamic management and recording of trial drugs, and improves the management level of clinical drug trials

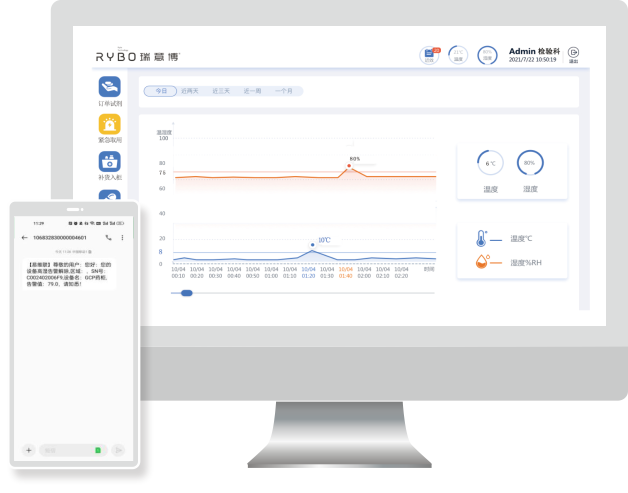
Inventory, expiration date, temperature and humidity early warning, access error alarm, drug isolation can be carried out after over temperature and humidity, and can be released from isolation or recovered after being evaluated by the sponsor; Outbound (return) operation is possible
Carry out coding management for each box of medicines, and record and trace the receiving, storage, distribution, dosage, dose, recovery, etc. of medicines throughout the process, support follow-up visits, and promote the standardization of clinical trial drug management


After intelligently checking the prescription and random order information, the corresponding drug is selected for distribution, and the drug distribution information is connected to the GCP drug management system in real time for accurate drug distribution and accurate traceability
Support one-key point and one-key query, without manual operation, save a lot of manpower and material resources, and reduce operating costs
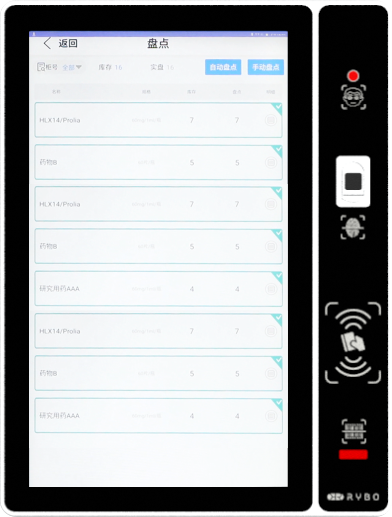
Make drug management more systematic, efficient and standardized |
The drug clinical trial process is traceable, and the authority management responsibilities are clear |
Prevent medication errors and improve medical efficiency |
WEBSITE MAP


Back Top